Construction Of A Voltalic Cells
20.3: Voltaic Cells
- Page ID
- 21792
- To understand the basics of voltaic cells
- To connect voltage from a voltaic cell to underlying redox chemistry
In whatever electrochemical process, electrons flow from one chemic substance to another, driven past an oxidation–reduction (redox) reaction. A redox reaction occurs when electrons are transferred from a substance that is oxidized to one that is being reduced. The reductant is the substance that loses electrons and is oxidized in the process; the oxidant is the species that gains electrons and is reduced in the process. The associated potential free energy is determined by the potential divergence between the valence electrons in atoms of unlike elements.
Because it is impossible to accept a reduction without an oxidation and vice versa, a redox reaction can be described as ii half-reactions, one representing the oxidation process and one the reduction process. For the reaction of zinc with bromine, the overall chemical reaction is as follows:
\[\ce{Zn(southward) + Br2(aq) \rightarrow Zn^{two+} (aq) + 2Br^{−} (aq)} \nonumber \]
The one-half-reactions are equally follows:
reduction one-half-reaction:
\[\ce{Br2 (aq) + 2e^{−} \rightarrow 2Br^{−} (aq)} \nonumber \]
oxidation one-half-reaction:
\[\ce{Zn (s) \rightarrow Zn^{2+} (aq) + 2e^{−} }\nonumber \]
Each half-reaction is written to testify what is actually occurring in the system; \(\ce{Zn}\) is the reductant in this reaction (it loses electrons), and \(\ce{Br2}\) is the oxidant (it gains electrons). Adding the two one-half-reactions gives the overall chemic reaction (Equation \(\PageIndex{1}\)). A redox reaction is counterbalanced when the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. Like any balanced chemical equation, the overall process is electrically neutral; that is, the net charge is the same on both sides of the equation.
In whatever redox reaction, the number of electrons lost by the oxidation reaction(s) equals the number of electrons gained by the reduction reaction(s).
In most of our discussions of chemical reactions, we have assumed that the reactants are in intimate concrete contact with one another. Acid–base reactions, for instance, are usually carried out with the acid and the base dispersed in a single phase, such every bit a liquid solution. With redox reactions, yet, information technology is possible to physically dissever the oxidation and reduction half-reactions in space, equally long equally there is a complete circuit, including an external electrical connexion, such as a wire, between the two half-reactions. As the reaction progresses, the electrons menses from the reductant to the oxidant over this electric connection, producing an electrical current that can be used to practice piece of work. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction is called an electrochemical cell.
There are two types of electrochemical cells: galvanic cells and electrolytic cells. Galvanic cells are named for the Italian physicist and physician Luigi Galvani (1737–1798), who observed that dissected frog leg muscles twitched when a small electric daze was practical, demonstrating the electric nature of nerve impulses. A galvanic (voltaic) cell uses the free energy released during a spontaneous redox reaction (\(ΔG < 0\)) to generate electricity. This type of electrochemical prison cell is ofttimes chosen a voltaic prison cell afterward its inventor, the Italian physicist Alessandro Volta (1745–1827). In dissimilarity, an electrolytic prison cell consumes electrical energy from an external source, using it to cause a nonspontaneous redox reaction to occur (\(ΔG > 0\)). Both types contain two electrodes, which are solid metals connected to an external circuit that provides an electrical connection between the two parts of the organization (Figure \(\PageIndex{1}\)). The oxidation half-reaction occurs at one electrode (the anode), and the reduction half-reaction occurs at the other (the cathode). When the circuit is closed, electrons flow from the anode to the cathode. The electrodes are also connected by an electrolyte, an ionic substance or solution that allows ions to transfer betwixt the electrode compartments, thereby maintaining the system's electrical neutrality. In this section, we focus on reactions that occur in galvanic cells.
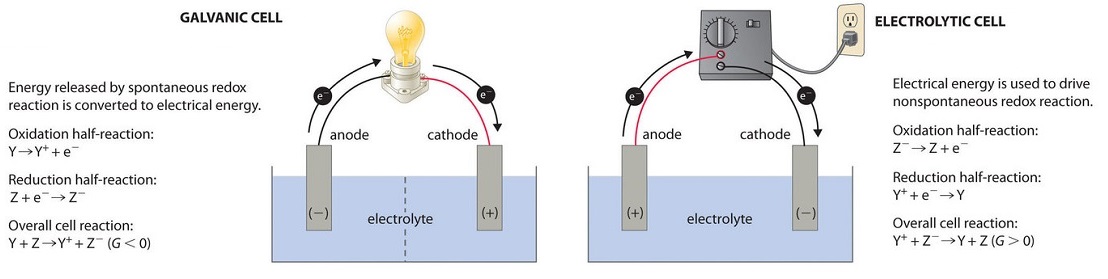
Voltaic (Galvanic) Cells
To illustrate the basic principles of a galvanic cell, permit's consider the reaction of metallic zinc with cupric ion (Cu2 +) to give copper metal and Zn2 + ion. The balanced chemical equation is as follows:
\[\ce{Zn (south) + Cu^{two+} (aq) \rightarrow Zn^{two+} (aq) + Cu(s)} \label{twenty.3.4} \]
We tin can cause this reaction to occur past inserting a zinc rod into an aqueous solution of copper(II) sulfate. Every bit the reaction proceeds, the zinc rod dissolves, and a mass of metallic copper forms. These changes occur spontaneously, but all the free energy released is in the course of rut rather than in a form that tin can be used to exercise work.
This same reaction can be carried out using the galvanic prison cell illustrated in Figure \(\PageIndex{3a}\). To assemble the cell, a copper strip is inserted into a chalice that contains a 1 M solution of \(\ce{Cu^{2+}}\) ions, and a zinc strip is inserted into a different beaker that contains a 1 M solution of \(\ce{Zn^{ii+}}\) ions. The ii metal strips, which serve every bit electrodes, are connected by a wire, and the compartments are connected by a salt bridge, a U-shaped tube inserted into both solutions that contains a concentrated liquid or gelled electrolyte. The ions in the table salt bridge are selected and so that they practice not interfere with the electrochemical reaction by being oxidized or reduced themselves or by forming a precipitate or circuitous; commonly used cations and anions are \(\ce{Na^{+}}\) or \(\ce{K^{+}}\) and \(\ce{NO3^{−}}\) or \(\ce{SO4^{2−}}\), respectively. (The ions in the common salt bridge do non take to be the aforementioned as those in the redox couple in either compartment.) When the circuit is closed, a spontaneous reaction occurs: zinc metallic is oxidized to \(\ce{Zn^{two+}}\) ions at the zinc electrode (the anode), and \(\ce{Cu^{two+}}\) ions are reduced to \(\ce{Cu}\) metal at the copper electrode (the cathode). As the reaction progresses, the zinc strip dissolves, and the concentration of \(\ce{Zn^{2+}}\) ions in the solution increases; simultaneously, the copper strip gains mass, and the concentration of \(\ce{Cu^{2+}}\) ions in the solution decreases (Figure \(\PageIndex{3b}\)). Thus we take carried out the same reaction every bit we did using a unmarried beaker, but this fourth dimension the oxidative and reductive half-reactions are physically separated from each other. The electrons that are released at the anode menstruum through the wire, producing an electric current. Galvanic cells therefore transform chemical free energy into electrical energy that can then be used to practice piece of work.
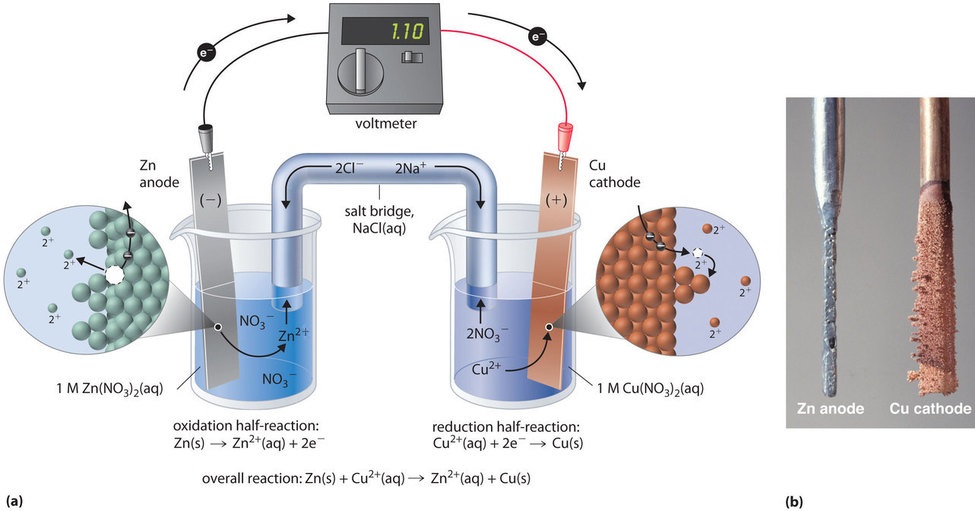
The electrolyte in the salt bridge serves two purposes: information technology completes the circuit by conveying electrical charge and maintains electrical neutrality in both solutions by allowing ions to migrate between them. The identity of the salt in a table salt bridge is unimportant, as long as the component ions do not react or undergo a redox reaction under the operating atmospheric condition of the cell. Without such a connexion, the full positive accuse in the \(\ce{Zn^{ii+}}\) solution would increase equally the zinc metallic dissolves, and the total positive charge in the \(\ce{Cu^{2+}}\) solution would subtract. The common salt bridge allows charges to exist neutralized by a flow of anions into the \(\ce{Zn^{2+}}\) solution and a menstruum of cations into the \(\ce{Cu^{two+}}\) solution. In the absence of a salt bridge or another similar connection, the reaction would quickly end because electrical neutrality could not be maintained.
A voltmeter tin be used to measure the difference in electric potential between the two compartments. Opening the switch that connects the wires to the anode and the cathode prevents a electric current from flowing, and then no chemic reaction occurs. With the switch airtight, still, the external circuit is closed, and an electric current can menstruum from the anode to the cathode. The potential (\(E_{cell}\)) of the cell, measured in volts, is the difference in electrical potential betwixt the 2 half-reactions and is related to the free energy needed to move a charged particle in an electrical field. In the cell we have described, the voltmeter indicates a potential of 1.10 5 (Figure \(\PageIndex{3a}\)). Because electrons from the oxidation half-reaction are released at the anode, the anode in a galvanic prison cell is negatively charged. The cathode, which attracts electrons, is positively charged.
Not all electrodes undergo a chemical transformation during a redox reaction. The electrode tin can be made from an inert, highly conducting metal such every bit platinum to prevent it from reacting during a redox procedure, where it does not appear in the overall electrochemical reaction. This miracle is illustrated in Example \(\PageIndex{1}\).
A galvanic (voltaic) prison cell converts the energy released by a spontaneous chemical reaction to electric energy. An electrolytic cell consumes electrical free energy from an external source to drive a nonspontaneous chemical reaction.
A chemist has constructed a galvanic prison cell consisting of two beakers. I chalice contains a strip of tin immersed in aqueous sulfuric acid, and the other contains a platinum electrode immersed in aqueous nitric acid. The two solutions are continued by a salt bridge, and the electrodes are continued by a wire. Electric current begins to menses, and bubbles of a gas appear at the platinum electrode. The spontaneous redox reaction that occurs is described by the following balanced chemic equation:
\[\ce{3Sn(southward) + 2NO3^{-}(aq) + 8H^{+} (aq) \rightarrow 3Sn^{two+} (aq) + 2NO (g) + 4H2O (l)} \nonumber \]
For this galvanic cell,
- write the half-reaction that occurs at each electrode.
- indicate which electrode is the cathode and which is the anode.
- betoken which electrode is the positive electrode and which is the negative electrode.
Given: galvanic jail cell and redox reaction
Asked for: one-half-reactions, identity of anode and cathode, and electrode assignment every bit positive or negative
Strategy:
- Identify the oxidation half-reaction and the reduction half-reaction. Then identify the anode and cathode from the one-half-reaction that occurs at each electrode.
- From the direction of electron flow, assign each electrode as either positive or negative.
Solution
A In the reduction half-reaction, nitrate is reduced to nitric oxide. (The nitric oxide would then react with oxygen in the air to form NOtwo, with its characteristic red-brown color.) In the oxidation half-reaction, metal tin is oxidized. The half-reactions corresponding to the actual reactions that occur in the organization are as follows:
reduction: \[\ce{NO3^{−} (aq) + 4H^{+}(aq) + 3e^{−} → NO(g) + 2H2O(l)} \nonumber \]
oxidation: \[\ce{Sn(south) → Sn^{2+}(aq) + 2e^{−}} \nonumber \]
Thus nitrate is reduced to NO, while the can electrode is oxidized to Sn2 +.
Because the reduction reaction occurs at the Pt electrode, it is the cathode. Conversely, the oxidation reaction occurs at the tin can electrode, and then information technology is the anode.
B Electrons flow from the tin electrode through the wire to the platinum electrode, where they transfer to nitrate. The electric excursion is completed by the salt span, which permits the diffusion of cations toward the cathode and anions toward the anode. Because electrons flow from the tin electrode, it must be electrically negative. In contrast, electrons menstruum toward the Pt electrode, and so that electrode must be electrically positive.
Consider a simple galvanic cell consisting of two beakers connected by a common salt bridge. Ane chalice contains a solution of \(\ce{MnO_4^{−}}\) in dilute sulfuric acid and has a Pt electrode. The other beaker contains a solution of \(\ce{Sn^{2+}}\) in dilute sulfuric acrid, also with a Pt electrode. When the two electrodes are connected by a wire, current flows and a spontaneous reaction occurs that is described by the following counterbalanced chemical equation:
\[\ce{2MnO^{−}4(aq) + 5Sn^{two+}(aq) + 16H^{+}(aq) \rightarrow 2Mn^{ii+}(aq) + 5Sn^{4+}(aq) + 8H2O(l)} \nonumber \]
For this galvanic cell,
- write the half-reaction that occurs at each electrode.
- point which electrode is the cathode and which is the anode.
- point which electrode is positive and which is negative.
- Answer a
-
\[\begin{align*} \ce{MnO4^{−}(aq) + 8H^{+}(aq) + 5e^{−}} &→ \ce{Mn^{two+}(aq) + 4H2O(50)} \\[4pt] \ce{Sn^{two+}(aq)} &→ \ce{Sn^{4+}(aq) + 2e^{−}} \terminate{marshal*} \nonumber \]
- Reply b
-
The Pt electrode in the permanganate solution is the cathode; the one in the tin solution is the anode.
- Answer c
-
The cathode (electrode in chalice that contains the permanganate solution) is positive, and the anode (electrode in beaker that contains the tin solution) is negative.
Electrochemical Cells: Electrochemical Cells(opens in new window) [youtu.be]
Amalgam Cell Diagrams (Prison cell Notation)
Because it is somewhat cumbersome to describe any given galvanic jail cell in words, a more convenient note has been developed. In this line notation, called a cell diagram, the identity of the electrodes and the chemical contents of the compartments are indicated by their chemic formulas, with the anode written on the far left and the cathode on the far right. Phase boundaries are shown by single vertical lines, and the common salt span, which has two phase boundaries, by a double vertical line. Thus the cell diagram for the \(\ce{Zn/Cu}\) jail cell shown in Figure \(\PageIndex{3a}\) is written equally follows:
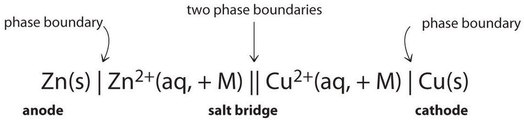
Galvanic cells can accept arrangements other than the examples we have seen so far. For example, the voltage produced past a redox reaction can be measured more accurately using two electrodes immersed in a single chalice containing an electrolyte that completes the circuit. This arrangement reduces errors caused past resistance to the flow of accuse at a purlieus, called the junction potential. One example of this blazon of galvanic cell is as follows:
\[\ce{Pt(s)\, | \, H2(one thousand) | HCl(aq, \, 1\,M)\,|\, AgCl(south) \,Ag(southward)} \nonumber \]
This cell diagram does not include a double vertical line representing a table salt bridge because there is no table salt bridge providing a junction between two dissimilar solutions. Moreover, solution concentrations have not been specified, so they are non included in the cell diagram. The half-reactions and the overall reaction for this jail cell are equally follows:
cathode reaction:
\[\ce{AgCl (southward) + e^{−} \rightarrow Ag(south) + Cl^{−}(aq)} \nonumber \]
anode reaction:
\[\ce{ 1/two H2(g) -> H^{+}(aq) + e^{-}} \nonumber \]
overall:
\[\ce{ AgCl(south) + i/2H2(thousand) -> Ag(s) + Cl^{-} + H^{+}(aq)} \nonumber \]
A single-compartment galvanic cell will initially exhibit the same voltage as a galvanic prison cell constructed using separate compartments, but it volition discharge rapidly because of the direct reaction of the reactant at the anode with the oxidized member of the cathodic redox couple. Consequently, cells of this blazon are not particularly useful for producing electricity.
Describe a cell diagram for the galvanic cell described in Example \(\PageIndex{1}\). The balanced chemic reaction is as follows:
\[\ce{3Sn(s) + 2NO^{−}iii(aq) + 8H^{+}(aq) \rightarrow 3Sn^{2+}(aq) + 2NO(g) + 4H2O(l)} \nonumber \]
Given: galvanic cell and redox reaction
Asked for: cell diagram
Strategy:
Using the symbols described, write the prison cell diagram beginning with the oxidation one-half-reaction on the left.
Solution
The anode is the tin strip, and the cathode is the \(\ce{Pt}\) electrode. Beginning on the left with the anode, we betoken the phase boundary between the electrode and the tin solution by a vertical bar. The anode compartment is thus \(\ce{Sn(due south)∣Sn^{two+}(aq)}\). We could include \(\ce{H2SO4(aq)}\) with the contents of the anode compartment, just the sulfate ion (as \(\ce{HSO4^{−}}\)) does not participate in the overall reaction, so it does non need to exist specifically indicated. The cathode compartment contains aqueous nitric acid, which does participate in the overall reaction, together with the product of the reaction (\(\ce{NO}\)) and the \(\ce{Pt}\) electrode. These are written as \(\ce{HNO3(aq)∣NO(yard)∣Pt(s)}\), with single vertical confined indicating the phase boundaries. Combining the two compartments and using a double vertical bar to bespeak the table salt bridge,
\[\ce{Sn(s)\,|\,Sn^{ii+}(aq)\,||\,HNO3(aq)\,|\,NO(g)\,|\,Pt_(due south)} \nonumber \]
The solution concentrations were not specified, so they are non included in this cell diagram.
Draw the cell diagram for the following reaction, assuming the concentration of \(\ce{Ag^{+}}\) and \(\ce{Mg^{2+}}\) are each ane Grand:
\[\ce{Mg(s) + 2Ag^{+}(aq) \rightarrow Mg^{2+}(aq) + 2Ag(south)} \nonumber \]
- Reply
-
\[ \ce{ Mg(due south) \,|\,Mg^{ii+}(aq, \;i \,M )\,||\,Ag^+(aq, \;ane\, M)\,|\,Ag(s)} \nonumber \]
Cell Diagrams: Prison cell Diagrams(opens in new window) [youtu.be]
Summary
A galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction to generate electricity, whereas an electrolytic cell consumes electric energy from an external source to strength a reaction to occur. Electrochemistry is the study of the relationship betwixt electricity and chemic reactions. The oxidation–reduction reaction that occurs during an electrochemical process consists of 2 half-reactions, 1 representing the oxidation process and one the reduction process. The sum of the one-half-reactions gives the overall chemical reaction. The overall redox reaction is balanced when the number of electrons lost by the reductant equals the number of electrons gained by the oxidant. An electric current is produced from the flow of electrons from the reductant to the oxidant. An electrochemical jail cell can either generate electricity from a spontaneous redox reaction or consume electricity to drive a nonspontaneous reaction. In a galvanic (voltaic) cell, the energy from a spontaneous reaction generates electricity, whereas in an electrolytic cell, electrical energy is consumed to drive a nonspontaneous redox reaction. Both types of cells utilise two electrodes that provide an electrical connection between systems that are separated in infinite. The oxidative half-reaction occurs at the anode, and the reductive one-half-reaction occurs at the cathode. A salt bridge connects the separated solutions, assuasive ions to migrate to either solution to ensure the system'due south electrical neutrality. A voltmeter is a device that measures the flow of electric electric current between two one-half-reactions. The potential of a cell, measured in volts, is the free energy needed to move a charged particle in an electrical field. An electrochemical cell can exist described using line note called a cell diagram, in which vertical lines indicate phase boundaries and the location of the common salt bridge. Resistance to the flow of charge at a boundary is called the junction potential.
Construction Of A Voltalic Cells,
Source: https://chem.libretexts.org/Bookshelves/General_Chemistry/Map:_Chemistry_-_The_Central_Science_%28Brown_et_al.%29/20:_Electrochemistry/20.03:_Voltaic_Cells
Posted by: washingtontured1978.blogspot.com


0 Response to "Construction Of A Voltalic Cells"
Post a Comment