Do Catalysts Affect Reaction Rate
What is a catalyst and what is catalysis?
Catalysts, past their definition, is a substance that increases the rate of a chemical reaction without changing themselves. The procedure by which a catalyst increases the rate of reaction is termed catalysis.
How practise catalysts work?
For reactants to react and give a product the reactant molecules must have threshold energy and the number of molecules with this free energy should also exist above a threshold value. This detail energy is chosen Activation Energy. Only those molecules from the reactants will be able to form products that have energy above activation energy.
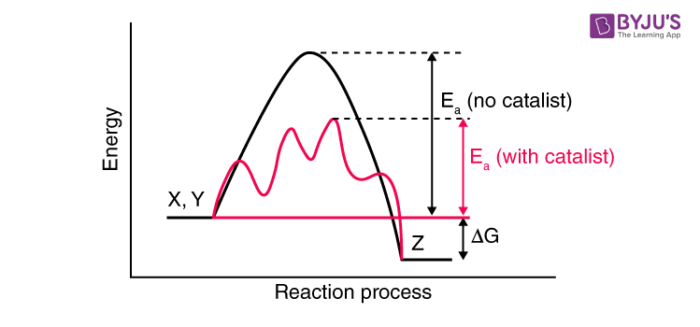
They change this activation energy or provide a dissimilar reaction mechanism that has a requirement of lower activation energy to requite products. The role of a goad in chemical reactions is best explained past intermediate-complex theory. Co-ordinate to intermediate-complex theory, information technology brings downward the activation energy for a reaction or provides a different pathway for the reaction where activation free energy is lower. It makes temporary bonds with the reactant molecules to grade an intermediate complex. This intermediate complex and so decomposes to give the products and the catalyst. The catalyst remains the same before and later the reaction. No change (chemical) is observed in them.
A catalyst tin can catalyze simply spontaneous reactions equally information technology cannot alter the Gibbs Free Energy, ΔG, and hence cannot catalyze a non-spontaneous reaction. A catalyst ever increases the charge per unit of a reaction; whatever substance that reduces the charge per unit of a reaction is called an inhibitor. Information technology has been observed that a catalyst does not change the equilibrium constant for a reaction but rather accelerates backwards as well as the frontward reaction to attain equilibrium fast. A catalyst catalyzes the forward besides as the astern reaction to the same extent and hence the equilibrium indicate remains the same and is attained fast as compared to the reaction without it.
Weather for catalysis:
Catalysts are highly efficient substances; a small corporeality of catalyst tin can catalyze a large number of reactants through catalysis. It is therefore important for the procedure designers to have ample information most the catalysts and the role they play in altering the reaction rates of diverse reactions. Catalysts take revolutionized the manufacturing manufacture. Near industries involved in manufacturing products and commodities use catalysts to reduce product costs and improve the rate of production. Even our torso has various types of catalysts that play an of import role in chemical reactions that occur inside our trunk, these are called enzymes. Catalysts play a very important function in today'southward earth and hence having some cognition about them will ever be helpful.
Oft Asked Questions – FAQs
How tin a positive catalyst alter the reaction?
A positive catalyst is to make the reaction rate very first by changing the path of reaction by decreasing the activation energy basis. Such that a large number of reactant molecules are converted into products.
What is the function of catalyst poison in Rosenmund reaction?
In the Rosenmund reaction aldehyde is prepared by reducing Acrid halides with hydrogen gas in the presence of palladium. If a goad is not poisoned the reaction is not stopped at the aldehyde level which is a feather reduction of alcohol. In order to cease at the aldehyde level. Palladium is poisoned with Barium sulphate.
What are the fundamental factors in heterogeneous catalysis?
In heterogeneous catalysis, the reacting and goad are in different states of thing. The about of import steps in this process are;
– Adsorption of reactant molecules activation centre.
– Formation of activation complex at the centre.
– This complex decomposes to give products.
– Desorption of products from the surface of the goad.
What is the office of promoters in Haber'due south procedure?
Promotors or accelerators increase the catalyst activity in a process. In Haber's process of manufacturing Ammonia, Nitrogen reacts with hydrogen to class NH3. Nitrogen is very less reactive and the yield of Ammonia is very less, to increase the percentage yield of Ammonia formed NO is used as a promoter.
What is the significance of autocatalysis?
Auto catalysis is self-catalysis in this process ane of the products formed acts as a catalyst and increases the reaction rate.
To know more about catalysts and catalysis, annals with BYJU'S.
Do Catalysts Affect Reaction Rate,
Source: https://byjus.com/chemistry/how-does-a-catalyst-affects-the-rate-of-chemical-reactions/
Posted by: washingtontured1978.blogspot.com


0 Response to "Do Catalysts Affect Reaction Rate"
Post a Comment