Who Discovered Strong Nuclear Force
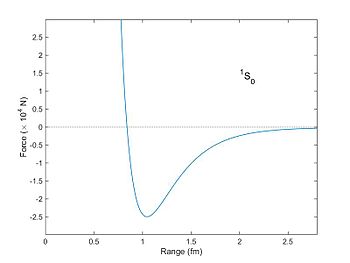
Strength (in units of x,000 Due north) between ii nucleons equally a function of distance as computed from the Reid potential (1968).[1] The spins of the neutron and proton are aligned, and they are in the Due south athwart momentum state. The bonny (negative) strength has a maximum at a altitude of about 1 fm with a force of about 25,000 N. Particles much closer than a distance of 0.8 fm feel a large repulsive (positive) force. Particles separated by a altitude greater than 1 fm are still attracted (Yukawa potential), but the force falls as an exponential function of altitude.
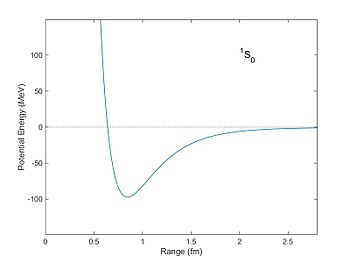
Respective potential energy (in units of MeV) of 2 nucleons as a function of distance as computed from the Reid potential. The potential well has a minimum at a distance of well-nigh 0.eight fm. With this potential nucleons can get leap with a negative "bounden energy".
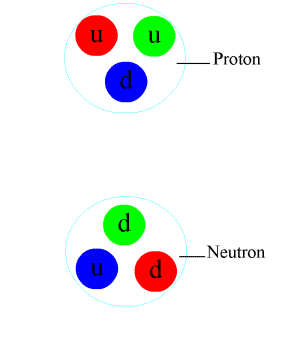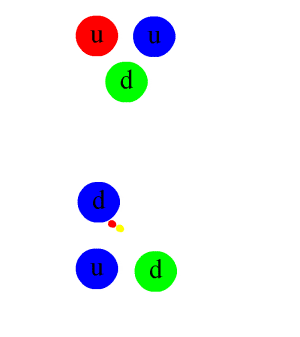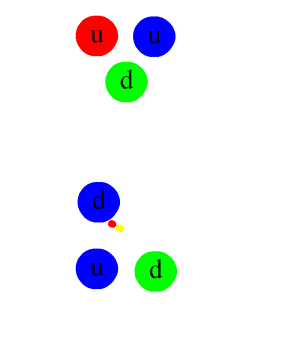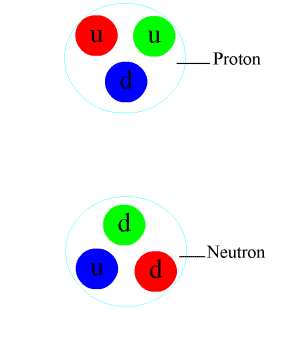
The nuclear strength (or nucleon–nucleon interaction, residual strong forcefulness, or, historically, stiff nuclear strength) is a force that acts between the protons and neutrons of atoms. Neutrons and protons, both nucleons, are affected by the nuclear force almost identically. Since protons have charge +ie, they experience an electric force that tends to push them autonomously, but at short range the attractive nuclear force is potent enough to overcome the electromagnetic force. The nuclear force binds nucleons into diminutive nuclei.
The nuclear force is powerfully bonny betwixt nucleons at distances of well-nigh 1 femtometre (fm, or 10−15 metre), but it rapidly decreases to insignificance at distances beyond near 2.five fm. At distances less than 0.vii fm, the nuclear force becomes repulsive. This repulsion is responsible for the size of nuclei, since nucleons can come no closer than the force allows. (The size of an atom, measured in angstroms (Å, or ten−10 m), is five orders of magnitude larger). The nuclear force is non simple, though, as it depends on the nucleon spins, has a tensor component, and may depend on the relative momentum of the nucleons.[2]
The nuclear force has an essential part in storing energy that is used in nuclear power and nuclear weapons. Work (energy) is required to bring charged protons together against their electric repulsion. This energy is stored when the protons and neutrons are bound together by the nuclear force to course a nucleus. The mass of a nucleus is less than the sum total of the private masses of the protons and neutrons. The departure in masses is known equally the mass defect, which can be expressed equally an free energy equivalent. Free energy is released when a heavy nucleus breaks apart into 2 or more than lighter nuclei. This energy is the electromagnetic potential free energy that is released when the nuclear force no longer holds the charged nuclear fragments together.[three] [4]
A quantitative description of the nuclear forcefulness relies on equations that are partly empirical. These equations model the internucleon potential energies, or potentials. (Generally, forces within a organisation of particles can be more simply modeled past describing the organisation'south potential energy; the negative gradient of a potential is equal to the vector force.) The constants for the equations are phenomenological, that is, determined by fitting the equations to experimental data. The internucleon potentials attempt to describe the properties of nucleon–nucleon interaction. In one case determined, whatsoever given potential tin be used in, eastward.g., the Schrödinger equation to determine the breakthrough mechanical properties of the nucleon organisation.
The discovery of the neutron in 1932 revealed that atomic nuclei were made of protons and neutrons, held together by an attractive force. By 1935 the nuclear strength was conceived to be transmitted by particles called mesons. This theoretical development included a clarification of the Yukawa potential, an early on example of a nuclear potential. Pions, fulfilling the prediction, were discovered experimentally in 1947. By the 1970s, the quark model had been developed, past which the mesons and nucleons were viewed as composed of quarks and gluons. By this new model, the nuclear force, resulting from the exchange of mesons betwixt neighboring nucleons, is a remainder effect[ vague ] of the strong force.
Description [edit]

Comparison betwixt the Nuclear Force and the Coulomb Forcefulness. a - remainder strong forcefulness (nuclear strength), rapidly decreases to insignificance at distances beyond about two.5 fm, b - at distances less than ~ 0.7 fm betwixt nucleons centers the nuclear strength becomes repulsive, c - coulomb repulsion force between ii protons (over 3 fm force becomes the master), d - equilibrium position for proton - proton, r - radius of a nucleon (a cloud composed of three quarks). Annotation: i fm = 1E-15 grand.
While the nuclear force is commonly associated with nucleons, more generally this force is felt between hadrons, or particles composed of quarks. At small separations between nucleons (less than ~ 0.7 fm betwixt their centers, depending upon spin alignment) the forcefulness becomes repulsive, which keeps the nucleons at a certain average separation. For identical nucleons (such as two neutrons or two protons) this repulsion arises from the Pauli exclusion force. A Pauli repulsion also occurs betwixt quarks of the same flavour from dissimilar nucleons (a proton and a neutron).
Field strength [edit]
At distances larger than 0.7 fm the forcefulness becomes attractive between spin-aligned nucleons, becoming maximal at a centre–center distance of nigh 0.9 fm. Across this distance the force drops exponentially, until across nigh 2.0 fm separation, the force is negligible. Nucleons have a radius of most 0.8 fm.[5]
At short distances (less than 1.7 fm or so), the attractive nuclear force is stronger than the repulsive Coulomb forcefulness between protons; it thus overcomes the repulsion of protons within the nucleus. However, the Coulomb forcefulness betwixt protons has a much greater range as information technology varies as the inverse square of the charge separation, and Coulomb repulsion thus becomes the only meaning force betwixt protons when their separation exceeds well-nigh 2 to ii.5 fm.
The nuclear strength has a spin-dependent component. The forcefulness is stronger for particles with their spins aligned than for those with their spins anti-aligned. If two particles are the same, such equally two neutrons or two protons, the force is not enough to bind the particles, since the spin vectors of two particles of the aforementioned type must point in opposite directions when the particles are well-nigh each other and are (save for spin) in the same quantum country. This requirement for fermions stems from the Pauli exclusion principle. For fermion particles of unlike types, such as a proton and neutron, particles may be close to each other and have aligned spins without violating the Pauli exclusion principle, and the nuclear strength may bind them (in this case, into a deuteron), since the nuclear forcefulness is much stronger for spin-aligned particles. Only if the particles' spins are anti-aligned the nuclear force is too weak to bind them, fifty-fifty if they are of different types.
The nuclear forcefulness also has a tensor component which depends on the interaction between the nucleon spins and the angular momentum of the nucleons, leading to deformation from a unproblematic spherical shape.
Nuclear binding [edit]
To disassemble a nucleus into unbound protons and neutrons requires piece of work against the nuclear force. Conversely, energy is released when a nucleus is created from free nucleons or other nuclei: the nuclear binding free energy. Considering of mass–energy equivalence (i.e. Einstein's formula E = mc 2 ), releasing this energy causes the mass of the nucleus to be lower than the total mass of the individual nucleons, leading to the so-called "mass defect".[6]
The nuclear force is nearly contained of whether the nucleons are neutrons or protons. This belongings is called accuse independence. The force depends on whether the spins of the nucleons are parallel or antiparallel, as it has a non-central or tensor component. This part of the force does not conserve orbital athwart momentum, which under the action of key forces is conserved.
The symmetry resulting in the potent force, proposed by Werner Heisenberg, is that protons and neutrons are identical in every respect, other than their charge. This is not completely true, considering neutrons are a tiny fleck heavier, but it is an approximate symmetry. Protons and neutrons are therefore viewed as the aforementioned particle, but with dissimilar isospin quantum numbers; conventionally, the proton is isospin up, while the neutron is isospin down. The strong strength is invariant under SU(2) isospin transformations, just as other interactions betwixt particles are invariant under SU(ii) transformations of intrinsic spin. In other words, both isospin and intrinsic spin transformations are isomorphic to the SU(ii) symmetry grouping. There are only strong attractions when the total isospin of the gear up of interacting particles is 0, which is confirmed by experiment.[7]
Our understanding of the nuclear forcefulness is obtained by scattering experiments and the binding energy of calorie-free nuclei.
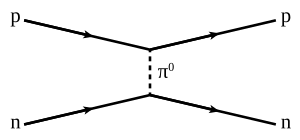
The nuclear force occurs by the exchange of virtual low-cal mesons, such as the virtual pions, every bit well as 2 types of virtual mesons with spin (vector mesons), the rho mesons and the omega mesons. The vector mesons account for the spin-dependence of the nuclear strength in this "virtual meson" flick.
The nuclear force is distinct from what historically was known equally the weak nuclear force. The weak interaction is one of the four central interactions, and plays a role in processes such as beta decay. The weak force plays no role in the interaction of nucleons, though it is responsible for the decay of neutrons to protons and vice versa.
History [edit]
The nuclear force has been at the heart of nuclear physics ever since the field was born in 1932 with the discovery of the neutron by James Chadwick. The traditional goal of nuclear physics is to empathize the backdrop of atomic nuclei in terms of the "bare" interaction between pairs of nucleons, or nucleon–nucleon forces (NN forces).
Within months later the discovery of the neutron, Werner Heisenberg[8] [9] [ten] and Dmitri Ivanenko[11] had proposed proton–neutron models for the nucleus.[12] Heisenberg approached the clarification of protons and neutrons in the nucleus through quantum mechanics, an approach that was not at all obvious at the time. Heisenberg's theory for protons and neutrons in the nucleus was a "major step toward understanding the nucleus as a quantum mechanical system".[13] Heisenberg introduced the first theory of nuclear exchange forces that bind the nucleons. He considered protons and neutrons to be unlike quantum states of the aforementioned particle, i.e., nucleons distinguished past the value of their nuclear isospin breakthrough numbers.
I of the earliest models for the nucleus was the liquid-drop model developed in the 1930s. I property of nuclei is that the average binding energy per nucleon is approximately the same for all stable nuclei, which is similar to a liquid drop. The liquid-drib model treated the nucleus as a drop of incompressible nuclear fluid, with nucleons behaving like molecules in a liquid. The model was get-go proposed by George Gamow and then adult by Niels Bohr, Werner Heisenberg, and Carl Friedrich von Weizsäcker. This crude model did not explain all the backdrop of the nucleus, just it did explicate the spherical shape of most nuclei. The model also gave good predictions for the binding energy of nuclei.
In 1934, Hideki Yukawa made the earliest endeavour to explain the nature of the nuclear force. According to his theory, massive bosons (mesons) mediate the interaction between 2 nucleons. In light of quantum chromodynamics (QCD)—and, past extension, the Standard Model—meson theory is no longer perceived equally primal. But the meson-exchange concept (where hadrons are treated as unproblematic particles) continues to represent the all-time working model for a quantitative NN potential. The Yukawa potential (also called a screened Coulomb potential) is a potential of the form
where g is a magnitude scaling constant, i.east., the amplitude of potential, is the Yukawa particle mass, r is the radial distance to the particle. The potential is monotone increasing, implying that the force is always attractive. The constants are determined empirically. The Yukawa potential depends simply on the distance r between particles, hence it models a central force.
Throughout the 1930s a group at Columbia Academy led by I. I. Rabi developed magnetic-resonance techniques to decide the magnetic moments of nuclei. These measurements led to the discovery in 1939 that the deuteron as well possessed an electric quadrupole moment.[xiv] [15] This electrical belongings of the deuteron had been interfering with the measurements by the Rabi group. The deuteron, composed of a proton and a neutron, is one of the simplest nuclear systems. The discovery meant that the physical shape of the deuteron was non symmetric, which provided valuable insight into the nature of the nuclear force bounden nucleons. In particular, the result showed that the nuclear force was not a fundamental force, but had a tensor character.[1] Hans Bethe identified the discovery of the deuteron's quadrupole moment equally i of the of import events during the formative years of nuclear physics.[14]
Historically, the job of describing the nuclear strength phenomenologically was formidable. The outset semi-empirical quantitative models came in the mid-1950s,[1] such as the Woods–Saxon potential (1954). In that location was substantial progress in experiment and theory related to the nuclear force in the 1960s and 1970s. 1 influential model was the Reid potential (1968)[1]
where and where the potential is given in units of MeV. In recent years,[ when? ] experimenters accept concentrated on the subtleties of the nuclear force, such every bit its charge dependence, the precise value of the πNN coupling constant, improved phase-shift analysis, high-precision NN data, high-precision NN potentials, NN handful at intermediate and high energies, and attempts to derive the nuclear force from QCD.[ citation needed ]
The nuclear force as a residuum of the stiff strength [edit]
An animation of the interaction. The colored double circles are gluons. Anticolors are shown every bit per this diagram (larger version).

The same diagram as that above with the individual quark constituents shown, to illustrate how the primal stiff interaction gives rising to the nuclear force. Straight lines are quarks, while multi-colored loops are gluons (the carriers of the fundamental forcefulness). Other gluons, which bind together the proton, neutron, and pion "in flying", are not shown.
The nuclear force is a residual consequence of the more fundamental strong forcefulness, or strong interaction. The stiff interaction is the attractive force that binds the unproblematic particles called quarks together to form the nucleons (protons and neutrons) themselves. This more powerful forcefulness, 1 of the fundamental forces of nature, is mediated by particles called gluons. Gluons hold quarks together through color charge which is analogous to electric accuse, but far stronger. Quarks, gluons, and their dynamics are mostly confined within nucleons, merely residual influences extend slightly beyond nucleon boundaries to give rising to the nuclear force.
The nuclear forces arising between nucleons are analogous to the forces in chemistry between neutral atoms or molecules called London dispersion forces. Such forces betwixt atoms are much weaker than the attractive electric forces that concord the atoms themselves together (i.e., that demark electrons to the nucleus), and their range between atoms is shorter, because they arise from pocket-sized separation of charges inside the neutral cantlet.[ further explanation needed ] Similarly, even though nucleons are made of quarks in combinations which abolish most gluon forces (they are "color neutral"), some combinations of quarks and gluons nevertheless leak away from nucleons, in the class of brusque-range nuclear force fields that extend from one nucleon to another nearby nucleon. These nuclear forces are very weak compared to direct gluon forces ("colour forces" or strong forces) inside nucleons, and the nuclear forces extend only over a few nuclear diameters, falling exponentially with distance. All the same, they are stiff plenty to bind neutrons and protons over short distances, and overcome the electrical repulsion between protons in the nucleus.
Sometimes, the nuclear force is called the residual strong force, in contrast to the stiff interactions which arise from QCD. This phrasing arose during the 1970s when QCD was being established. Earlier that time, the strong nuclear force referred to the inter-nucleon potential. After the verification of the quark model, strong interaction has come to mean QCD.
Nucleon–nucleon potentials [edit]
Ii-nucleon systems such every bit the deuteron, the nucleus of a deuterium atom, as well as proton–proton or neutron–proton scattering are ideal for studying the NN force. Such systems can exist described by attributing a potential (such every bit the Yukawa potential) to the nucleons and using the potentials in a Schrödinger equation. The form of the potential is derived phenomenologically (by measurement), although for the long-range interaction, meson-exchange theories help to construct the potential. The parameters of the potential are determined past fitting to experimental information such as the deuteron binding energy or NN elastic scattering cantankerous sections (or, equivalently in this context, then-called NN phase shifts).
The about widely used NN potentials are the Paris potential, the Argonne AV18 potential,[sixteen] the CD-Bonn potential and the Nijmegen potentials.
A more recent arroyo is to develop constructive field theories for a consistent clarification of nucleon–nucleon and iii-nucleon forces. Quantum hadrodynamics is an effective field theory of the nuclear forcefulness, comparable to QCD for color interactions and QED for electromagnetic interactions. Additionally, chiral symmetry breaking can be analyzed in terms of an effective field theory (called chiral perturbation theory) which allows perturbative calculations of the interactions between nucleons with pions as exchange particles.
From nucleons to nuclei [edit]
The ultimate goal of nuclear physics would be to describe all nuclear interactions from the basic interactions between nucleons. This is called the microscopic or ab initio approach of nuclear physics. At that place are two major obstacles to overcome:
- Calculations in many-trunk systems are difficult (because of multi-particle interactions) and require advanced computation techniques.
- In that location is bear witness that three-nucleon forces (and possibly college multi-particle interactions) play a significant role. This means that three-nucleon potentials must be included into the model.
This is an active area of research with ongoing advances in computational techniques leading to better showtime-principles calculations of the nuclear shell structure. Ii- and iii-nucleon potentials take been implemented for nuclides up to A = 12.
Nuclear potentials [edit]
A successful way of describing nuclear interactions is to construct 1 potential for the whole nucleus instead of considering all its nucleon components. This is called the macroscopic approach. For example, scattering of neutrons from nuclei can be described by because a plane wave in the potential of the nucleus, which comprises a real part and an imaginary part. This model is often called the optical model since information technology resembles the instance of light scattered by an opaque glass sphere.
Nuclear potentials can be local or global: local potentials are limited to a narrow energy range and/or a narrow nuclear mass range, while global potentials, which have more parameters and are ordinarily less authentic, are functions of the energy and the nuclear mass and can therefore be used in a wider range of applications.
See likewise [edit]
-
 Physics portal
Physics portal - Nuclear binding free energy
References [edit]
- ^ a b c d Reid, R. V. (1968). "Local phenomenological nucleon–nucleon potentials". Annals of Physics. 50 (three): 411–448. Bibcode:1968AnPhy..fifty..411R. doi:ten.1016/0003-4916(68)90126-7.
- ^ Kenneth S. Krane (1988). Introductory Nuclear Physics. Wiley & Sons. ISBN0-471-80553-X.
- ^ Binding Energy, Mass Defect, Hirsuite Elephant physics educational site, retrieved 2012-07-01.
- ^ Affiliate four. NUCLEAR PROCESSES, THE Stiff Strength, M. Ragheb, 1/30/2013, University of Illinois.
- ^ Povh, B.; Rith, K.; Scholz, C.; Zetsche, F. (2002). Particles and Nuclei: An Introduction to the Concrete Concepts. Berlin: Springer-Verlag. p. 73. ISBN978-3-540-43823-6.
- ^ Stern, Dr. Swapnil Nikam (Feb 11, 2009). "Nuclear Binding Free energy". From Stargazers to Starships. NASA website. Retrieved 2010-12-30 .
- ^ Griffiths, David, Introduction to Elementary Particles
- ^ Heisenberg, Due west. (1932). "Über den Bau der Atomkerne. I". Z. Phys. (in High german). 77 (1–2): one–11. Bibcode:1932ZPhy...77....1H. doi:x.1007/BF01342433. S2CID 186218053.
- ^ Heisenberg, W. (1932). "Über den Bau der Atomkerne. 2". Z. Phys. (in German). 78 (three–four): 156–164. Bibcode:1932ZPhy...78..156H. doi:ten.1007/BF01337585. S2CID 186221789.
- ^ Heisenberg, West. (1933). "Über den Bau der Atomkerne. Iii". Z. Phys. (in German). 80 (ix–10): 587–596. Bibcode:1933ZPhy...80..587H. doi:10.1007/BF01335696. S2CID 126422047.
- ^ Iwanenko, D. D., The neutron hypothesis, Nature 129 (1932) 798.
- ^ Miller A. I. Early Quantum Electrodynamics: A Sourcebook, Cambridge University Printing, Cambridge, 1995, ISBN 0521568919, pp. 84–88.
- ^ Brown, L. M.; Rechenberg, H. (1996). The Origin of the Concept of Nuclear Forces. Bristol and Philadelphia: Constitute of Physics Publishing. ISBN0750303735.
- ^ a b John S. Rigden (1987). Rabi, Scientist and Citizen. New York: Basic Books, Inc. pp. 99–114. ISBN9780674004351 . Retrieved May ix, 2015.
- ^ Kellogg, J. One thousand.; Rabi, I. I.; Ramsey, N. F.; Zacharias, J. R. (1939). "An electrical quadrupole moment of the deuteron". Physical Review. 55 (3): 318–319. Bibcode:1939PhRv...55..318K. doi:10.1103/physrev.55.318. Retrieved May ix, 2015.
- ^ Wiringa, R. B.; Stoks, Five. G. J.; Schiavilla, R. (1995). "Accurate nucleon–nucleon potential with charge-independence breaking". Physical Review C. 51 (1): 38–51. arXiv:nucl-thursday/9408016. Bibcode:1995PhRvC..51...38W. doi:10.1103/PhysRevC.51.38. PMID 9970037. S2CID 118937727.
Bibliography [edit]
- Gerald Edward Dark-brown and A. D. Jackson (1976). The Nucleon–Nucleon Interaction. Amsterdam Northward-Kingdom of the netherlands Publishing. ISBN 0-7204-0335-9.
- R. Machleidt and I. Slaus, "The nucleon–nucleon interaction", J. Phys. One thousand 27 (May 2001) R69. doi:10.1088/0954-3899/27/v/201. (Topical review.)
- E. A. Nersesov (1990). Fundamentals of atomic and nuclear physics. Moscow: Mir Publishers. ISBN 5-06-001249-ii.
- Navrátil, Petr; Ormand, Due west. Erich (2003). "Ab initio trounce model with a genuine 3-nucleon strength for the p-shell nuclei". Physical Review C. 68 (3): 034305. arXiv:nucl-th/0305090. Bibcode:2003PhRvC..68c4305N. doi:10.1103/PhysRevC.68.034305. S2CID 119091461.
Further reading [edit]
- Ruprecht Machleidt, "Nuclear Forces", Scholarpedia, 9(one):30710. doi:10.4249/scholarpedia.30710.
Who Discovered Strong Nuclear Force,
Source: https://en.wikipedia.org/wiki/Nuclear_force
Posted by: washingtontured1978.blogspot.com






0 Response to "Who Discovered Strong Nuclear Force"
Post a Comment